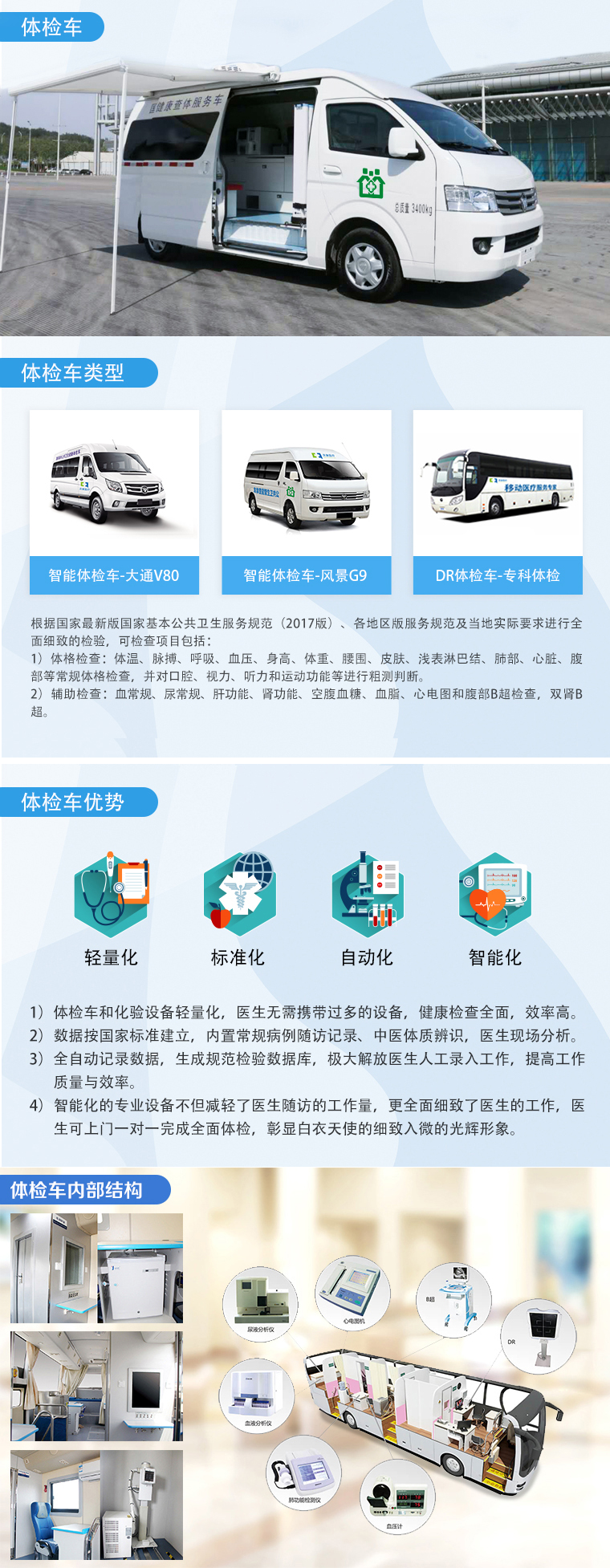
實(shí)施案例
產(chǎn)品推薦
新聞推薦
基本公共衛(wèi)生體檢系統(tǒng)
- 2022-11-09
- http://www.gzdyynz.com/ 原創(chuàng)
- 130
1. GMP registration consultation is the embodiment of total quality management in the pharmaceutical industry& lt; Regulations for the Implementation of the Standardization Law of the People's Republic of China& gt; Article 18 stipulates that "standards and industrial standards are divided into mandatory standards and recommendatory standards". Drug standards are mandatory.
2、產(chǎn)品質(zhì)量咨詢的種類按質(zhì)量咨詢的責(zé)任不同,可分為自我咨詢、使用方咨詢、第三方咨詢。按咨詢內(nèi)容不同,可分為質(zhì)量咨詢、體系咨詢、安全咨詢。藥品關(guān)系人命安危,因此藥品咨詢屬于安全咨詢,是屬于一種強(qiáng)制性的咨詢。
2. The types of product quality consultation can be divided into self consultation, user consultation and third-party consultation according to the different responsibilities of quality consultation. According to different consultation contents, it can be divided into quality consultation, system consultation and safety consultation. Drugs are related to the safety of human life, so drug consultation is a kind of safety consultation and a kind of mandatory consultation.
3、GMP是一部體現(xiàn)質(zhì)量管理和質(zhì)量保證新概念的國際GMP,其特點(diǎn)體現(xiàn)在它是結(jié)合iso9000~9004標(biāo)準(zhǔn)系列修改而成的標(biāo)準(zhǔn)。而在國外有些執(zhí)行著美國FDA咨詢的標(biāo)準(zhǔn),我國也有些單位通過了美國FDA咨詢。
3. GMP is an international GMP that embodies the new concept of quality management and quality assurance, and its characteristics are embodied in that it is a modified standard combined with iso9000~9004 standard series. While in foreign countries, some units implement the standards of FDA consultation in the United States, and in China, some units have passed the FDA consultation in the United States.
4、國際咨詢的意義本身就是不僅要加強(qiáng)藥廠內(nèi)部諸多質(zhì)量因素的過程控制,也要對藥廠外部關(guān)鍵質(zhì)量因素有所控制。如配方、原料、輔料、包裝材料、儀器設(shè)備以及建筑材料的質(zhì)量采取控制措施。
4. The significance of international consultation is not only to strengthen the process control of many quality factors inside the pharmaceutical factory, but also to control the key quality factors outside the pharmaceutical factory. Take control measures for the quality of formula, raw materials, auxiliary materials, packaging materials, instruments and equipment and building materials.
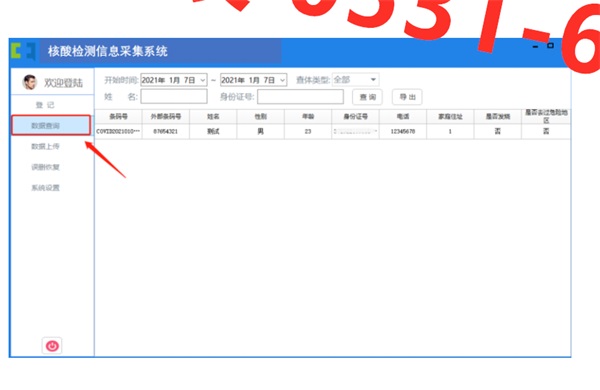

5、藥品監(jiān)督管理局是代表對藥品獨(dú)立地進(jìn)行第三方公正評價(jià)的GMP咨詢機(jī)構(gòu),其代碼C12。負(fù)責(zé)國際藥品貿(mào)易中優(yōu)先采購、使用推薦、優(yōu)先受理新藥藥品申請。迄至1998年6月31日末取得咨詢的企業(yè),藥品監(jiān)督管理部門將不再受理新藥生產(chǎn)的申請。
5. The Drug Administration is a GMP consulting agency that independently conducts a third-party fair evaluation of drugs, its code is C12. Be responsible for giving priority to purchase, use and recommendation, and receiving new drug applications in international drug trade. For enterprises that have obtained consultation by the end of June 31, 1998, the drug regulatory authorities will no longer accept applications for new drug production.
6、GMP注冊咨詢是集軟件、硬件、安全、衛(wèi)生、環(huán)保于一身的強(qiáng)制性咨詢,那么它就必須建立和運(yùn)行著科學(xué)的、公認(rèn)的國際管理體系,要請有資歷的第二方(咨詢機(jī)構(gòu))匯同本企業(yè)專家進(jìn)行整體策劃、評估,制訂出適合本企業(yè)(含國際標(biāo)準(zhǔn)、標(biāo)準(zhǔn)、行業(yè)標(biāo)準(zhǔn))規(guī)范的質(zhì)量管理手冊及作業(yè)指導(dǎo)書,在學(xué)習(xí)、培訓(xùn)、運(yùn)轉(zhuǎn)的不斷修正過程中,再來申請GMP咨詢才是每個(gè)藥業(yè)人明智的選擇。
6. GMP registration consultation is a mandatory consultation integrating software, hardware, safety, health and environmental protection. Therefore, it must establish and operate a scientific and recognized international management system. A qualified second party (consulting agency) should be invited to participate in the overall planning and evaluation together with the enterprise's experts, so as to develop a quality management manual and operation instructions suitable for the enterprise's (including international standards, standards and industrial standards) specifications In the process of continuous correction of operation, it is a wise choice for every pharmaceutical company to apply for GMP consultation again.
以上就是為大家介紹的有關(guān)公共衛(wèi)生體檢檢管理系統(tǒng)的詳細(xì)的介紹,希望對您有所幫助.如果您有什么疑問的話,歡迎聯(lián)系我們.我們將以專業(yè)的態(tài)度,為您提供服務(wù)http://www.gzdyynz.com/
The above is a detailed introduction to the management system of public health physical examination. I hope it will be helpful to you. If you have any questions, please contact us. We will provide you with professional services http://www.gzdyynz.com/